2: Air pollutants
Pollutants can be of two types : Primary and secondary . Let's look deeper into the origin of these pollutants.
The pollutants present in the air can be in the form of solid particles, liquid/droplets or gaseous form. The quality of urban air is indicated by the quantity of certain pollutants in the air, like Ozone (O₃), Particulate Matter (PM₁₀, PM₂.₅), Sulphur Oxides (SOₓ), Nitrogen Oxides (NOₓ), Carbon monoxide (CO) and Volatile Organic Compounds (VOCs). The pollutants are classified into two categories on the basis of their origin-

Primary Pollutants
The pollutants that are emitted from both natural and anthropogenic sources directly into the atmosphere are referred to as ‘primary pollutants’. These pollutants are a matter of concern as they are not only harmful for humans and the environment but also contribute to the formation of secondary pollutants. The primary pollutants are further classified into following categories:
* Particulate Matter
Particulate matter (PM), or particle pollution, refers to the mixture of solid and liquid particles in the air. It includes particles that are large enough to be viewed with the naked eye and small particles that require a microscope for detection. These are very fine particles that can penetrate deep into our lungs due to their small size. They are classified based on the size of the particles. The two major categories of PM are:
- PM 10: inhalable particles with a diameter of ≤10 micrometres
- PM 2.5: fine inhalable particles with a diameter of ≤2.5 micrometres
Source: Dust from construction sites, industrial sources, landfills, agricultural sites, highways etc. Anthropogenic sources include agricultural operations, industrial processes, combustion of wood and fossil fuels, construction/demolition activities, and dusty roads and natural sources include windblown dust, volcanoes etc.
Effect: Due to their small size, they enter the blood through the lungs and have negative consequences on health, including lung cancer, respiratory illnesses, and cardiovascular problems. They also produce haze, which impairs visibility and impacts the vegetation.
* Oxides of Sulfur (SOx)
Oxides of sulphur include gases like sulphur dioxide (SO2) and sulphur trioxide (SO3), which are formed when the sulphur-containing compounds undergo combustion in the presence of plenty of oxygen. These are colourless gases found in the lower atmosphere but can react and emit harmful odours. Other lower oxides are relatively less stable in the atmosphere compared to sulphur dioxide and trioxide.
Source: Sulphur dioxide (SO2) is produced from volcanic eruptions, copper metallurgy, vehicular emissions, the burning of fossil fuels (coal), etc.
Sulphur trioxide (SO3) is produced in the sulphuric acid manufacturing process.
Effect: The oxides of sulphur cause respiratory disorders like asthma and bronchitis, leading to skin burns and other internal organ damage when inhaled, and contribute to the formation of acid rain in the atmosphere, affecting all lifeforms and property on the planet. Also, it contributes to the formation of particulate pollution in the atmosphere.
* Oxides of Nitrogen (NOx)
Oxides of Nitrogen i.e, Nitric Oxide (NO) and Nitrogen dioxide (NO2) are formed from the interaction of nitrogen and oxygen. Nitric Oxides are flammable, odourless gas, while nitrogen dioxides are inflammable, poisonous reddish- orange coloured gas. In ambient conditions, Nitrogen Oxides are rapidly oxidised in air to form nitrogen dioxide.
Source: Natural sources include volcanoes, biological decay, lightning/ thunder and oceans. Power plants, vehicular emissions, industrial/ domestic combustion are few of the anthropogenic sources of the oxides of nitrogen.
Effect: When inhaled, it disturbs the protective mucous lining of the lungs. Long term exposure to high concentrations may result in eyes, nose, throat and respiratory tract irritation and chronic lung disorders. Also, it affects plant and animal life. In the presence of sunlight, it aids in the formation of photochemical smog.
* Carbon Monoxide (CO)
Carbon monoxide is a colourless, odourless, tasteless, and toxic air pollutant. It is produced as a result of partial/ incomplete combustion of carbon containing substances like fossil fuels etc. Vehicular emissions are one of the major contributors to this pollutant in the atmosphere, especially in cooler conditions as the automobile efficiency decreases at lower temperatures.
Source: Natural sources include volcanoes, wildfires, natural gas in coaleries, and marsh gases (plants in waterlogged conditions). While, the industrial emissions, automobile emissions, certain products (cigarettes, lawn mowers), and inefficient functioning machinery and equipment are examples of anthropogenic sources.
Effects: It contributes indirectly to global warming and climate change by influencing the amount of greenhouse gases (GHGs) in the atmosphere. In the case of inhalation by humans, it interacts with haemoglobin and reduces the oxygen-carrying capacity of the blood. Thus, it causes flu-like symptoms, dizziness, confusion, headaches, chest pains, and may even lead to death.
* Volatile Organic Compounds (VOCs)
Emitted as gases from different solid and liquid products, VOCs, include a variety of chemicals that result in adverse effects on health and environment. They cause both indoor and outdoor pollution. The concentration of VOCs is recorded to be higher indoors compared to outdoors. This is because they are majorly included in household products and cause harm to indoor air quality
Source: Natural sources comprise emissions from plants, natural forest fires etc. The anthropogenic sources include:
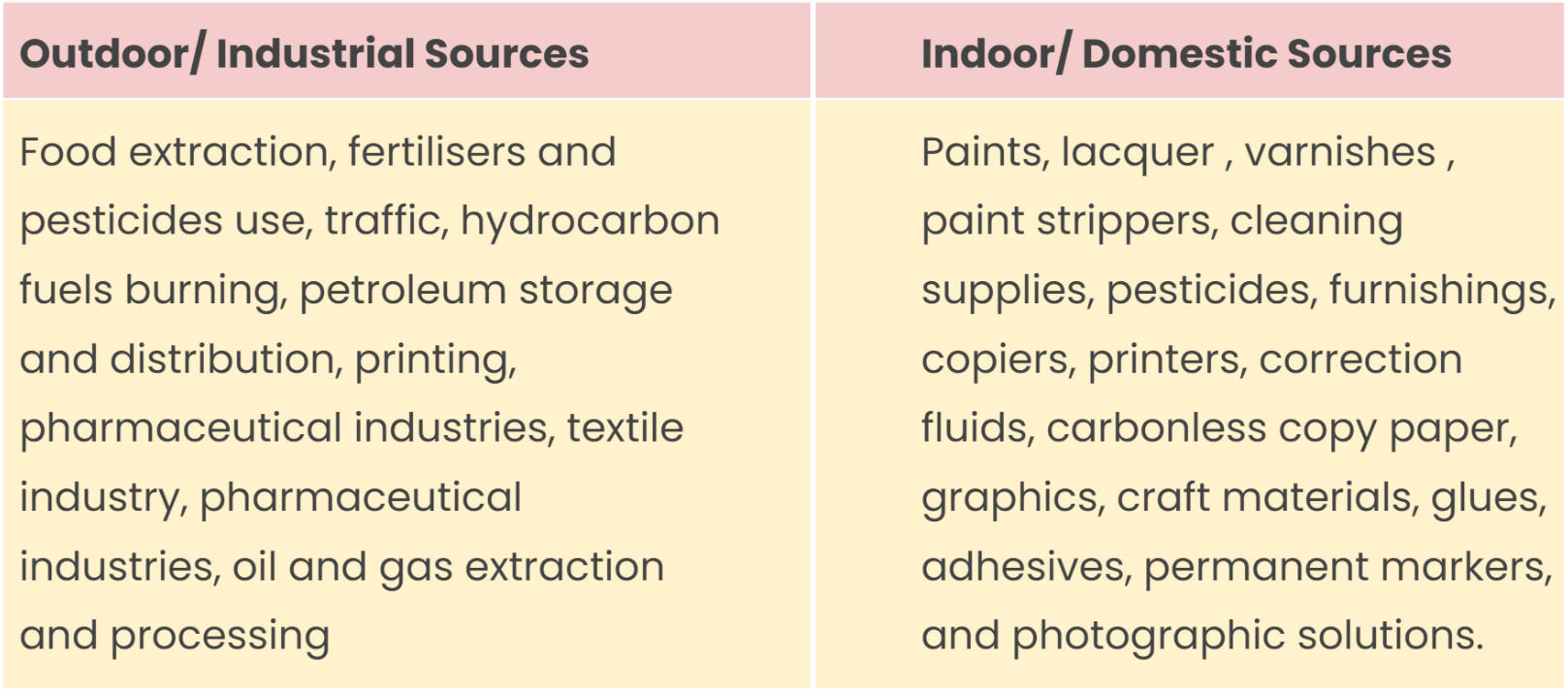
Effects: VOCs are corrosive and thus damage materials and products, result in the formation of photochemical smog, directly contribute to global warming, and also cause ozone depletion. In humans, they cause eye, nose, and throat irritation, difficult breathing, and nausea. They can even damage the central nervous system and other organs. Some of the VOCs are known to be carcinogenic.
Secondary Pollutants
Unlike the primary pollutants, these pollutants are not emitted into the atmosphere directly. They are formed as a result of interaction between the primary two or more primary pollutants in the atmosphere. These pollutants are alarming as they are formed from different compounds present in the atmosphere and thus are more harmful. Some of the primary pollutants (like nitrogen oxides, particulate matter etc.) can be formed as a result of interactions in the atmosphere, and thus can be considered as secondary pollutants. The following are secondary pollutants:
i) Smog
The word "smog" is a combination of smoke and fog. It is often used as a generic term for any kind of air pollution that reduces visibility, especially in urban areas. Broadly, it can be distinguished as industrial smog and photochemical smog.
- Photochemical Smog: It is formed as a consequence of interaction of VOCs, Nitrogen dioxide, ozone, PAN in the presence of ample sunlight. This occurs when there is very low movement of air.
- Industrial Smog: Also known as grey or black smog, develops under cold and humid conditions which are often associated with inversions that trap the pollution near the earth’s surface. It is caused due to burning of fossil fuels (coal), triggering the release of smoke and sulphur dioxide. These react with fog particles and create red haze, i.e., smog.
Example- Delhi smog is best example of both photochemical and industrial smog
Effects: Increase in allergies, coughing, and severe irritation of eyes, nose, and throat. Increase in the chance of developing birth defects, lung disorders etc.
ii) Peroxyacetyl Nitrate (PAN)
PAN is a secondary pollutant that is formed in the atmosphere due to the interaction between VOCs and nitrogen dioxide. It is one of the major components of photochemical smog. It can persist for a longer period of time in the atmosphere under cool conditions.
Effects: If humans are exposed to PAN for longer durations, it may cause severe eye irritation and respiratory disorders. Also, it shows adverse effects on the crop plants and other vegetation.
iii) Acid Rain
The oxides of nitrogen and sulphur interact with the water vapour present in the atmosphere. The end result of this interaction is the formation of nitric acid (HNO3) and sulphuric acid (H2S04). The acid formed falls on the earth’s surface along with precipitation. Therefore, it is termed as acid rain.
Example- yellowing of historical monument, the Taj Mahal
Effects: It causes skin burns, irritation of eyes and nose, cardio-vascular and respiratory disorders in humans. It makes the soil and water acidic, disturbing the natural processes of the environment. It does not only impact all life forms on the planet, but also destroys materials/ buildings.
iv) Ozone
Ozone in the stratosphere is beneficial for humans as it prevents the entry of harmful UV rays from the sun into the earth. The ground- level ozone, present in the troposphere, acts as a pollutant. It is formed from the interaction of two primary pollutants, VOCs and NOX. It is a highly irritating colourless gas.
Effects: It triggers a variety of health problems including chest pain, coughing, throat irritation, congestion, asthma. It also causes damage to the plantation and decreases its productivity.